
by Grow Up Conference | Apr 16, 2024 | Cannabis News Wire, Media Partners
Florida is witnessing a surge in momentum as it gears up for what is anticipated to be the costliest recreational cannabis legalization campaign in American history. Led by Trulieve Cannabis Corp. (CSE: TRUL) (OTCQX: TCNNF), based in Tallahassee, the campaign is attracting support from six other multistate cannabis operators (MOS), marking a significant expansion in the effort to transition Florida’s thriving $2 billion medical cannabis market, currently the largest in the nation, into one that caters to recreational use too.
The latest supporters joining forces with Trulieve include prominent players in the cannabis industry such as Ayr Wellness, Cresco Labs, Curaleaf Holdings, Green Thumb Industries, Insa and Verano Holdings.
The committee behind the initiative — Smart and Safe Florida — announced a substantial boost to its funding, with an additional $15 million raised. With the new support, the campaign currently has a total of approximately $55 million in funding. Trulieve alone had already raised and spent more than $40 million by the end of last year.
For perspective, backers of Ohio’s initiative, Issue 2, spent less than $7 million, while California’s Proposition 64, a victorious recreational use legislation, saw expenditures reach $25 million in 2016.
The recent endorsement from major marijuana businesses comes on the heels of a significant legal victory at the state’s Supreme Court, where a constitutional challenge mounted by Ashley Moody, the state attorney general, was dismissed. The decision paves the way for the initiative to proceed, providing a boost to the campaign’s momentum.
Should the initiative, called Amendment 3, be adopted by 60% of state voters in the November elections, it would permit currently operating medical cannabis treatment centers to begin selling recreational marijuana by May 2025. Additionally, it seeks to allow adults 21 years of age and older to possess up to three ounces while maintaining the prohibition on home cultivation, consistent with existing state laws.
However, the measure does not include a social equity scheme, and the state legislature would still need to take action to expand licenses beyond the current operators. Presently, the state hosts 627 dispensaries operated by 25 firms, with regulators indicating the possibility of issuing permits to 22 more by summer, albeit at a notably high license renewal fee of $1.33 million.
Florida’s market potential is enormous given its 22 million citizens, 859,000-plus registered medical marijuana patients and millions of tourists each year. Estimates suggest that the state’s $2 billion in yearly medical cannabis sales might double.
Even though a recent poll shows significant voter support for recreational cannabis (62%), it is expected that more funding would be needed to organize a successful statewide campaign. On top of Trulieve’s $40 million investment, estimates indicate that total campaign expenses including media buys and operational costs may total between $20 million and $25 million.
About CNW420
CNW420 spotlights the latest developments in the rapidly evolving cannabis industry through the release of an article each business day at 4:20 p.m. Eastern – a tribute to the time synonymous with cannabis culture. The concise, informative content serves as a gateway for investors interested in the legalized cannabis sector and provides updates on how regulatory developments may impact financial markets. If marijuana and the burgeoning industry surrounding it are on your radar, CNW420 is for you! Check back daily to stay up-to-date on the latest milestones in the fast -changing world of cannabis.
To receive SMS alerts from CNW, text CANNABIS to 888-902-4192 (U.S. Mobile Phones Only)
For more information, please visit https://www.CannabisNewsWire.com
Please see full terms of use and disclaimers on the CannabisNewsWire website applicable to all content provided by CNW, wherever published or re-published: https://www.CannabisNewsWire.com/Disclaimer
CannabisNewsWire
Denver, CO
www.CannabisNewsWire.com
303.498.7722 Office
Editor@CannabisNewsWire.com
CannabisNewsWire is powered by IBN

by Grow Up Conference | Apr 16, 2024 | Grow Opportunity, Media Partners
(Globe Newswire) Toronto — Heritage Cannabis Holdings Corp. announced the commencement of a sale and investment solicitation process in respect of the business and assets of the company, 1005477 B.C. Ltd., Heritage Cannabis West Corporation, Mainstrain Market Ltd., Heritage Cannabis East Corporation, Purefarma Solutions Inc., 333 Jarvis Realty Inc., 5450 Realty Inc., Heritage Cannabis Exchange Corp. and Premium 5 Ltd. The SISP will be conducted in the Heritage Group’s ongoing proceedings under the Companies’ Creditors Arrangement Act.
On April 2, 2024, the Heritage Group obtained an initial order from the Ontario Superior Court of Justice granting the Heritage Group protection under the CCAA. The Initial Order appointed KPMG Inc. as the Court-appointed monitor of the Heritage Group. The Initial Order also extended certain protections to, among others, the Heritage Group’s subsidiaries in the United States of America.
On April 11, 2024, an order approving the SISP was granted by the Court, authorizing the Monitor to undertake the SISP for the sale of the Heritage Group’s (i) property, assets and undertaking or shares in the capital of one or more of the Heritage Group entities, and (ii) business operations. In addition, the SISP Order approved a stalking horse subscription agreement among the company and Heritage West, as vendors, BJK Holdings Ltd., and HAB Cann Holdings Ltd., for the purpose of serving as the stalking horse bidder in the context of the SISP, in order to establish the baseline consideration for the company’s business and assets. A copy of the SISP is attached to the SISP Order. The SISP Order is available on the Monitor’s Website (as defined below).
The SISP will be administered by KPMG Inc., in its capacity as the Monitor of the Heritage Group, with the assistance of the Heritage Group’s management team. The SISP is intended to solicit interest in and opportunities for a sale of, or investment in, all or part of the Heritage Group’s Property and Business. This may include one or more of a restructuring, refinancing, recapitalization or other form of reorganization of the Business and affairs of one or more entities comprising the Heritage Group as a going concern, or a sale of all, substantially all, or one or more components of the Heritage Group’s Property and Business as a going concern or otherwise.
All qualified interested parties will be provided with an opportunity to participate in the SISP, including receipt of a process summary describing the opportunity and access to a virtual data-room, which will be made available upon the execution of a non-disclosure agreement acceptable to the Heritage Group and the Monitor. The deadline to submit a Sale Proposal, Partial Sale Proposal, or an Investment Proposal (as such terms are defined in the SISP), as the case may be, to the Monitor in accordance with the terms of the SISP is set for 5:00 p.m. EST on May 10, 2024.

by Grow Up Conference | Apr 16, 2024 | Cannabis News Wire, Media Partners
- Lexaria, a global innovator in drug delivery platforms, has added three new patents to its portfolio, bringing the total to 41
- Two patents were awarded in the U.S., while the third was awarded in Japan, expiring in 2042 and 2041, respectively, if not extended
- Lexaria’s management plans to maintain focus on GLP-1 studies for 2024
Lexaria Bioscience (NASDAQ: LEXX), a global innovator in drug delivery platforms, in the month of April 2024, added three new patents to its portfolio. This brings the total number of awarded patents globally to 41, a testament to the versatility of its patented DehydraTECH(TM) technology and its overall viability in the potential treatment of various ailments including, but not limited to, diabetes, weight loss and hypertension.
Two newly granted patents were awarded in the U.S. They fall under Lexaria’s patent family #24: Compositions and Methods for Treating Epilepsy. These two patents complement earlier research that discovered DehydraTECH-CBD was capable of mitigating epileptic seizures in animals, and was also absorbed into the bloodstream more effectively than the commercially available CBD-based anti-seizure medication, Epidiolex(R).
The third patent was granted in Japan under Lexaria’s patent family #18: Compositions and Methods for Enhanced Delivery of Antiviral Agents. This patent followed Lexaria’s demonstration of a 42%-204% improved delivery of antiviral drugs, as evidenced in numerous animal studies over the years. The first two patents will expire in 2042, while the last one will expire in 2041 if both are not extended.
These three additions bolster Lexaria’s intellectual property, which remains significant in supporting future business objectives. They are also integral in growing the company’s shareholder value, an avenue it seeks to pursue even further as time progresses. Even so, Lexaria is still committed to focusing on glucagon-like peptide 1 (“GLP-1”) studies for the 2024 calendar year, having shown positive results in an 8-week clinical study in 2023. At the start, the company’s foray into GLP-1 studies was considered a “high-risk” program, according to the CEO, Chris Bunka. This mainly stemmed from the fact that this was a class of drugs considered “large molecules,” a class that Lexaria had never explored before, given its focus on “small molecules” (https://cnw.fm/ITon1).
Despite the challenges and concerns, Lexaria’s GLP-1 study was a huge success that aligned with the rising interest in GLP-1 drugs owing to their health benefits. As such, the company looks to concentrate on these studies, even as it grows its intellectual property globally.
“Our R&D plans for 2024 are very tightly focused and will be concentrated mainly on GLP-1 investigations. We are not at this time planning additional 2024 research in the antiviral nicotine, or PDE5 sectors. We have solid early-stage data in each of those areas that will allow us to build upon those at the right time,” noted Mr. Bunka.
GLP-1 studies will run alongside international patent applications. Upcoming patents are expected to cover swallowed capsules and dissolvable oral tablets. Lexaria’s management is confident in its current direction and maintains that 2024 will be the year the company’s hard work will prove itself. It is a testament to its commitment to creating shareholder value and asserting itself as a leader in its space.
For more information, visit the company’s website at www.LexariaBioscience.com.
NOTE TO INVESTORS: The latest news and updates relating to LEXX are available in the company’s newsroom at https://cnw.fm/LEXX
About CannabisNewsWire
CannabisNewsWire (“CNW”) is a specialized communications platform with a focus on cannabis news and the cannabis sector. It is one of 60+ brands within the Dynamic Brand Portfolio @ IBN that delivers: (1) access to a vast network of wire solutions via InvestorWire to efficiently and effectively reach a myriad of target markets, demographics and diverse industries; (2) article and editorial syndication to 5,000+ outlets; (3) enhanced press release enhancement to ensure maximum impact; (4) social media distribution via IBN to millions of social media followers; and (5) a full array of tailored corporate communications solutions. With broad reach and a seasoned team of contributing journalists and writers, CNW is uniquely positioned to best serve private and public companies that want to reach a wide audience of investors, influencers, consumers, journalists and the general public. By cutting through the overload of information in today’s market, CNW brings its clients unparalleled recognition and brand awareness. CNW is where breaking news, insightful content and actionable information converge.
To receive SMS alerts from CNW, text CANNABIS to 888-902-4192 (U.S. Mobile Phones Only)
For more information, please visit https://www.CannabisNewsWire.com
Please see full terms of use and disclaimers on the CannabisNewsWire website applicable to all content provided by CNW, wherever published or re-published: https://www.CannabisNewsWire.com/Disclaimer
CannabisNewsWire
Denver, CO
www.CannabisNewsWire.com
303.498.7722 Office
Editor@CannabisNewsWire.com
CannabisNewsWire is powered by IBN

by Grow Up Conference | Apr 16, 2024 | Cannabis Prospect Magazine, Media Partners

by Grow Up Conference | Apr 16, 2024 | Cannabis Prospect Magazine, Media Partners

by Grow Up Conference | Apr 16, 2024 | Cannabis Prospect Magazine, Media Partners

by Grow Up Conference | Apr 16, 2024 | Cannabis Prospect Magazine, Media Partners

by Grow Up Conference | Apr 16, 2024 | Media Partners, Psychedelic News Wire
A recently published review has determined that using psilocybin to manage mental-health conditions doesn’t heighten the risk of paranoia. This discovery comes at a time when public interest in the therapeutic potential of psychedelics is increasing.
The review was carried out by researchers at Larkin University, the University of Georgia and Palm Beach Atlantic University, who were focused on better understanding the potential negative effects of psilocybin therapy.
For their study, the researchers reviewed six randomized, double-blind clinical trials with a total of 528 patients, where psilocybin was administered to treat depression and anxiety. They used ClinicalTrials.gov, Web of Science and MEDLINE to look for publications available between 1966 and November 2023. Of the total number of participants, 51% were female, with a median age of 39.8 years.
The researchers discovered that psilocybin wasn’t linked to a risk of paranoia and transient thought disorder but noted that rare cases needed to be monitored over the long-term. However, they did identify other adverse effects among patients in the trials, which included nausea, headache, dizziness, increased blood pressure and anxiety. In their report, the researchers noted that the adverse effects of a single therapeutic dose of psilocybin appeared to be resolved and tolerable in 48 hours.
They also highlighted the need for future research to more actively assess the appropriate management of these effects. They added that the effectiveness of alternative therapies in managing mental-health conditions needed to be further studied and noted that the role licensed therapists played in managing effects observed also presented an avenue for future study.
The review was published in “JAMA Psychiatry” by the American Medical Association. Researchers involved in the study include Akhila Yerubandi, N. M. Mahmudul Alam Bhuiya, Jennifer E. Thomas, Lorenzo Villa Zapata, Catherine Harrington and Joshua Caballero.
In other news, the American Medical Association published separate research in March that contradicted common beliefs on the possible risks associated with the use of psychedelics by adolescents. This research determined that psychedelics were linked to lower rates of psychotic symptoms in the target population. Additionally, clinical-trial results published late last year suggested that psilocybin-assisted psychotherapy was safe and effective in treating bipolar II disorder. This condition has been linked to difficult-to-treat and debilitating depressive episodes.
Furthermore, the association published a study in August 2023 which determined that after receiving one dose of psilocybin, individuals with major depressive disorder experienced clinically sustained reduction in their symptoms.
Psychedelics have been found to have immense therapeutic potential, and companies such as Seelos Therapeutics Inc. (NASDAQ: SEEL) are focused on developing psychedelic treatments to help patients suffering from many conditions that currently lack effective medications. The JAMA study indicating that the risk of paranoia isn’t amplified when psilocybin is used in the treatment of mental health conditions is a welcome finding since it allays the fears that some people may have had.
About PsychedelicNewsWire
PsychedelicNewsWire (“PNW”) is a specialized communications platform with a focus on all aspects of psychedelics and the latest developments and advances in the psychedelics sector. It is one of 60+ brands within the Dynamic Brand Portfolio @ IBN that delivers: (1) access to a vast network of wire solutions via InvestorWire to efficiently and effectively reach a myriad of target markets, demographics and diverse industries; (2) article and editorial syndication to 5,000+ outlets; (3) enhanced press release enhancement to ensure maximum impact; (4) social media distribution via IBN to millions of social media followers; and (5) a full array of tailored corporate communications solutions. With broad reach and a seasoned team of contributing journalists and writers, PNW is uniquely positioned to best serve private and public companies that want to reach a wide audience of investors, influencers, consumers, journalists and the general public. By cutting through the overload of information in today’s market, PNW brings its clients unparalleled recognition and brand awareness. PNW is where breaking news, insightful content and actionable information converge.
To receive SMS alerts from PsychedelicNewsWire, text “Groovy” to 888-902-4192 (U.S. Mobile Phones Only)
For more information, please visit https://www.PsychedelicNewsWire.com
Please see full terms of use and disclaimers on the PsychedelicNewsWire website applicable to all content provided by PNW, wherever published or re-published: https://www.PsychedelicNewsWire.com/Disclaimer
PsychedelicNewsWire
San Francisco, CA
www.PsychedelicNewsWire.com
415.949.5050 Office
Editor@PsychedelicNewsWire.com
PsychedelicNewsWire is powered by IBN

by Grow Up Conference | Apr 16, 2024 | Garden Culture Magazine, Media Partners
There’s a lot of buzz about regenerative gardening these days, and for good reason. After all, regenerative gardening techniques help heal the planet by building healthy soil and conserving natural resources like water and energy. The regenerative gardener never uses chemicals and works exclusively with nature to create a low-maintenance, sustainable ecosystem that offers harvests and wildlife habitats. You’ll find yourself craving time in your garden and learn that if you were to walk away from it, the garden would continue to thrive and regenerate without you, like a forest.
So, are you ready to become a regenerative grower? There’s nothing overly complicated about it; there are easy, practical steps you can take immediately to get started.
Low-Mow Lawns, Lasagna Gardens, and Hugelkultur
Saying goodbye to chemical sprays is the first thing you can do. After that, observe your space and consider different actions you can take. Do you have sections of lawn you can replace with wildflowers or low-mow alternatives? Can you build soil by creating a lasagna garden or hugelkultur mound? These two options use easy-to-find organic materials and can even be applied to containers! They act like sponges and retain moisture, reducing the need for watering as your plants grow.


Conserving Natural Resources
Find ways to integrate water catchment systems into your space, whether a rain garden, rain barrel, or garden ollas. Make use of rock walls, concrete spaces, or the exterior walls of your home that retain warmth and place heat-loving crops close by.


Boost Soil Health
Amend soil with compost and green manures, and always cover the earth with natural mulches. Remember, the key is to mimic what you see in nature. Forests and meadows feature plants that grow intensively and in succession, have different bloom times, hardly any pest issues, and mulch the soil with fallen leaves and other debris. By selecting a wide variety of native perennial plants for your space, you’re copying what happens in the natural environment around us. This is regenerative gardening.


Welcome Critters and Community
Incorporating bug and worm hotels, bat and bird houses, and spots with clean water for birds and bees to drink and bathe lets our creature friends know they’re welcome to take refuge in our garden spaces. They’re also fantastic focal points in a pollinator victory garden that will allow you to observe the wildlife living in your area.
The regenerative garden is inclusive, and encouraging your community to jump on the bandwagon is part of the fun. Consider setting up a neighborhood seed library, cut flower stand, and sensory spaces for everyone to enjoy.


Settle into the Groove
Take baby steps as you implement eco-friendly practices into your growing regime. Once settled, you’ll find the garden requires little intervention from you. Gardening doesn’t have to be constant work; you can do this. We wouldn’t throw anything at you that we didn’t think you couldn’t do.
Good Resources
Stephanie Rose (of Garden Therapy) has written two incredible books about creating regenerative garden spaces. I encourage you to read Garden Alchemy: 80 Recipes and Concoctions for Organic Fertilizers, Plant Elixrs, Potting Mixes, Pest Detterents, and more, and my personal favorite, The Regenerative Garden: 80 Practical Projects for Creating a Self-Sustaining Garden Ecosystem.
These two guidebooks are perfect for gardeners of all skill levels and will set you on the right track for a productive, zero-work, regenerative garden.
Remember, regenerative gardening might seem like a new concept, but there’s nothing novel about it. Take Mother Nature’s lead in your garden space, and everything will fall into place.
What’s your favorite thing about your regenerative garden? Let us know in the comments below!
by Grow Up Conference | Apr 16, 2024 | Extraction Magazine, Media Partners
Bioavailability refers to how much (quantity) and how quickly (rate) an active substance (drug) absorbs and becomes available at the target site in the body. [1] From another perspective, it is the percent of a substance that reaches systemic circulation. An intravenous injection (IV) has a bioavailability of 100%: the substance is injected into the bloodstream instantly and made completely available. So IVs get an A+ for bioavailability.
Non-hospital administration methods fall elsewhere on the curve. These include CBD/cannabis inhalation, nasal spray, transdermal applications, and oils/tinctures/edibles. These administration routes put obstacles in the way of absorption. In the case of oil-based orals, the hurdles are significant.
Cannabinoids & Bioavailability
Oral administration takes CBD/cannabis on a long journey. First, must navigate through the intestinal wall. Then they enter portal circulation, where they are beaten into submission (metabolized) by the liver. First-pass metabolism characterizes this great sojourn; at each stage, cannabinoids are rendered inactive and effectively useless. These inactive metabolites are then excreted by the kidneys… or simply wholesale. [2,3] But it gets worse.
From the start, cannabinoids were ill-equipped for the trip. The body readily absorbs water-soluble compounds. The water we drink boasts near 100% bioavailability. Cannabinoids are not water soluble. On the contrary, they are highly lipophilic, meaning they attract and dissolve in lipids/fats. Oil and water don’t play well together. Of course, the body does absorb lipids, but the process requires bile secretion and micelle formation. The result is that most cannabinoids cannot muster a sufficient rate of absorption before clearance.
Terrible absorption rates do not bode well for bioavailability:
- Oral THC bioavailability has been estimated at 4-20%. [2,4]
- Oral CBD bioavailability has been estimated at 13-19%. [3]
To put this in perspective, the bioavailability of THC inhalation has been estimated as high as 56%. [4] Average bioavailability from CBD inhalation has been estimated at 31 %. [3] Robust research is limited regarding bioavailability, especially in humans. Still, oral ingestion gets a solid D for bioavailability.
More on Tinctures
Tinctures represent the lion’s share of CBD products. [5] Tinctures aim to boost bioavailability with a simple instruction: hold the oil under your tongue for 60 seconds or longer. In general, the highly-vascularized tissue under the tongue (sublingual) is purported to afford 3-10 times greater absorption compared to simply swallowing. [6]
So What Stands as a Problem?
The problem is that available research (which is limited) suggests that bioavailability for oral cannabinoids does not significantly improve (statistically) with sublingual or oro-mucosal administration. [2-4, 6-8] Enhanced absorption from the sublingual route remains a hypothesis for cannabinoids. Perhaps this administration method extends to the upper range of the estimated oral bioavailability (19-20%). That is also a hypothesis!
Now we’ve discovered a sobering fact: oral bioavailability of cannabinoids is poor. On an A-F scale, we gave it a D.
But as Albert Einstein said, “Reality is merely an illusion, albeit a very persistent one.” Fortunately, it’s possible to bend the reality of oral bioavailability.
Combining a high-fat meal with cannabinoids produces major improvements in bioavailability. Zgair et al (2016) observed a 2.5- to 3-fold increase in systemic exposure of THC and CBD when administered to rats with sesame oil (mostly long-chain triglycerides). [6] A 2019 study published in Epilepsia examined eight human patients; a high-fat meal prior to CBD capsules amplified maximum concentration 14-fold and area-under-the-curve 4-fold. [7]
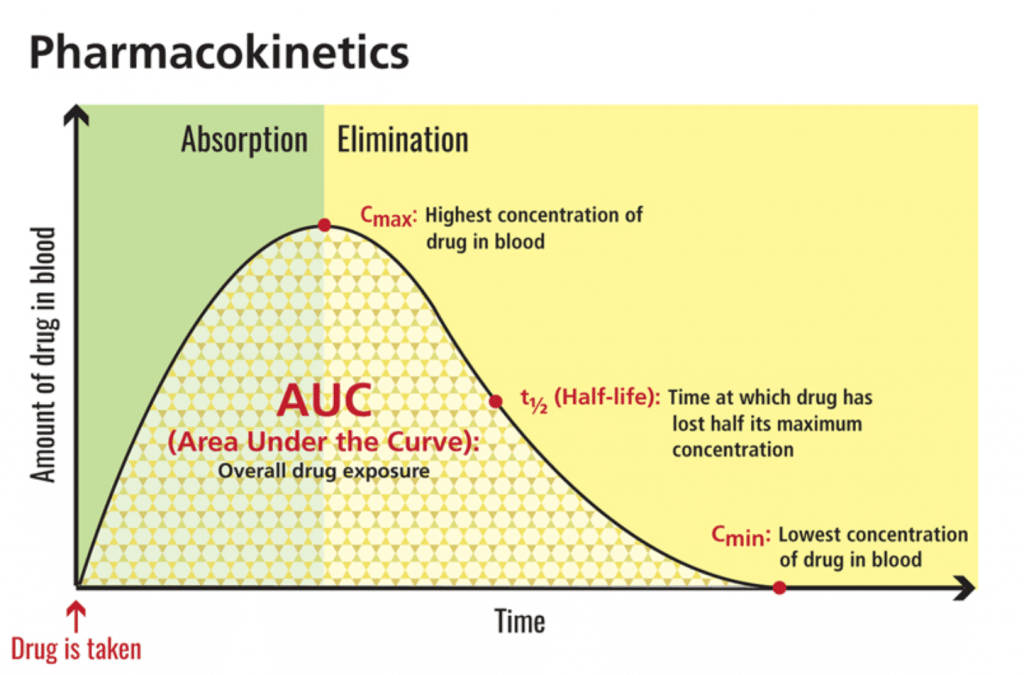
Maximum concentration, or cmax, refers to the highest amount of a substance (i.e., CBD) that reaches circulation. Area-under-the-curve, or AUC, reflects total exposure over time. In this 2019 study, CBD not only reached levels 14 times higher than fasted baseline — it also multiplied total exposure by four. [7] In terms of bioavailability, this is a big deal.
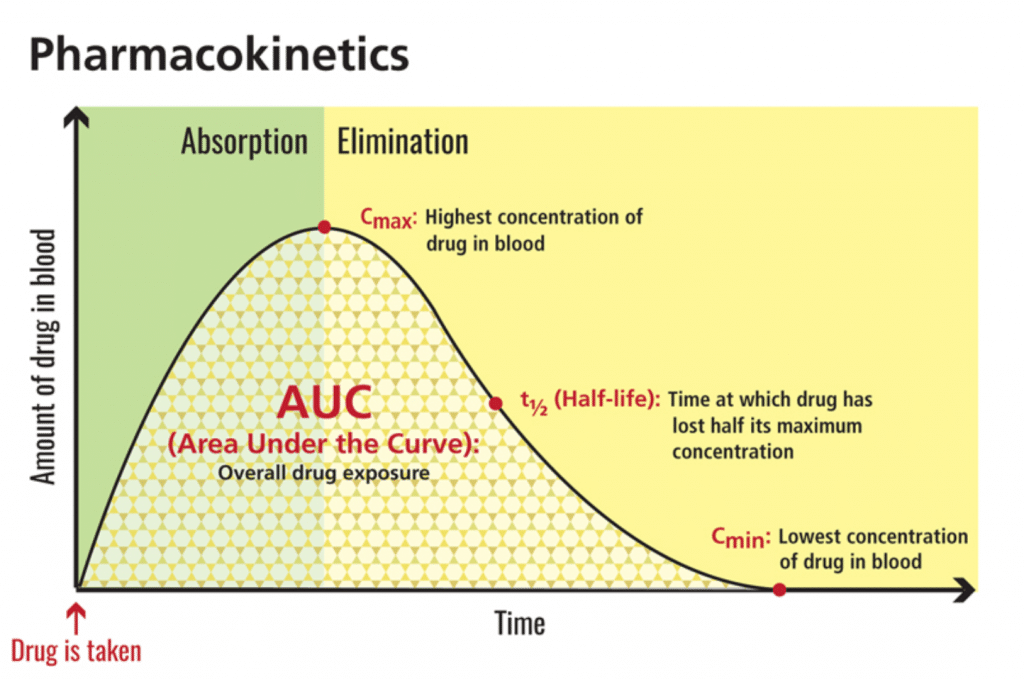
[Graphic 2: U.S. National Library of Medicine]
The FDA-approved CBD isolate drug Epidiolex® echoes this phenomenon. Look no further than the safety label: “Coadministration of EPIDIOLEX with a high-fat/high-calorie meal increased Cmax by 5-fold, AUC by 4-fold, and reduced the total variability, compared with the fasted state in healthy volunteers.”
This Bioavailability Graph Miracle
When ingested, fats are broken down and form chylomicrons (CM), or lipid-protein particles. These particles have strong affinity for cannabinoids. Cannabinoids jump on the CM bus, so to speak. Those that make it (an estimated 1/3) bypass first-pass metabolism. They enter circulation through intestinal lymphatic transport. [6] Instead of portal circulation and relentless pounding from the liver, these cannabinoids enter circulation through lymph. [8]
This is not the same as simply infusing cannabinoids in oil carriers. The purpose of extraction/ into fats is decarboxylation and solubility. Achieving peak CM levels appears to require significant fat content, such as that found in medium- to high-fat meals. [6,9]
Another promising adventure concerns liposomal formulations. Liposomes are lipid nanoparticles that serve as carriers or transporters for a whole range of pharmaceuticals. [8,10] Essentially, they are phospho-lipid ‘bubbles’ capable of delivering encased lipids (e.g., cannabinoids) into aqueous solutions. This gives cannabinoids the surface appearance of being “water-soluble” although they are not.
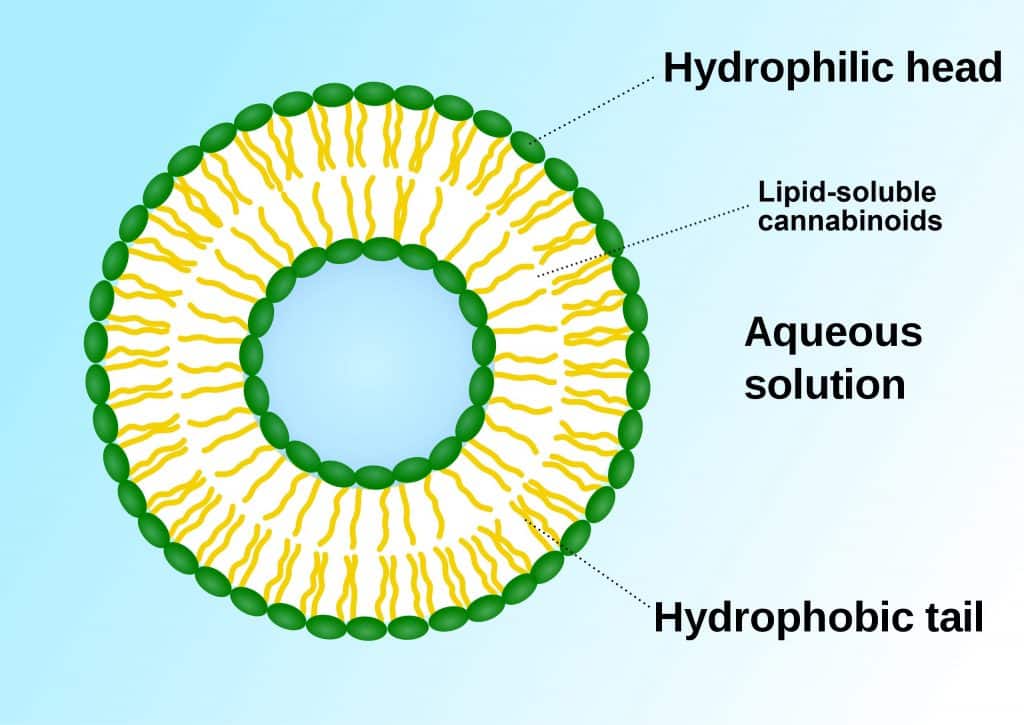

[Graphic 3: SuperManu]
Remember, cannabinoids are fat-loving, water-hating molecules. The phospho-lipids in a liposome have hydrophilic (water-loving) heads and hydrophobic (water-hating) tails. The bi-layer (two layers back-to-back) means lipids can be trapped inside. Liposomes may thereby help protect cannabinoids from first-pass metabolism and increase rates of absorption substantially. It also appears that liposomes increase CM and lymphatic transport (like high-fat meals) although this mechanism of action is unclear. [8]
Recently, several companies have patented liposomal cannabinoid products designed to enhance absorption. Marketing that says ‘water-soluble’ likely relates to liposomes; cannabinoids are never water soluble, but may appear as such when encased in a liposome. Challenges associated with this technology include manufacturing difficulties (such as drug loading), variable stability, and cost. [10]
Final Word
The marketing surrounding cannabinoid products can be dizzying. Terms like enhanced absorption, superior delivery, and even water-soluble all point to bioavailability. Overall, bioavailability is how much of the cannabinoids we dose actually reach the bloodstream. Due to their lipophilic nature, oral cannabinoids receive a D in bioavailability. But a high-fat meal or liposomal technology can boost this grade up to a B. And that’s worth pinning on the fridge.
References
- Chow, Shein-Chung. “Bioavailability and Bioequivalence in Drug Development.” Wiley Interdisciplinary Reviews: Computational Statistics,6, no.4, 2014, pp. 304-312, doi:10.1002/wics.1310. Journal Impact Factor = 1.75, Times Cited = 11 (ResearchGate)
- Mcgilveray, Iain J. “Pharmacokinetics of Cannabinoids.” Pain Research and Management, vol. 10, suppl. a, 2005, doi:10.1155/2005/242516. Journal Impact Factor = 1.685, Times Cited = 72 (ResearchGate)
- Millar, Sophie A., et al. “A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans.” Frontiers in Pharmacology, vol. 9, 2018, doi:10.3389/fphar.2018.01365. Journal Impact Factor = 3.831, Times Cited = 10 (ResearchGate)
- Huestis, Marilyn A. “Human Cannabinoid Pharmacokinetics.” Chemistry & Biodiversity, 4, no. 8, 2007, pp. 1770-804. doi:10.1002/cbdv.200790152. Journal Impact Factor = 1.449, Times Cited = 284 (ResearchGate)
- Corroon, Jamie, and Joy A. Phillips. “A Cross-Sectional Study of Cannabidiol Users.” Cannabis and Cannabinoid Research, vol. 3, no. 1, 2018, pp. 152–161, doi:10.1089/can.2018.0006. Journal Impact Factor = NA, Times Cited = 12 (ResearchGate)
- Narang, N. & Jyoti Sharma. “Sublingual Mucosa as a Route for Systemic Drug Delivery.” International Journal of Pharmacy and Pharmaceutical Sciences, 3, no.2, 2011, pp. 18-22. Journal Impact Factor = 4.773, Times Cited = 65
- Schoedel, Kerri A., and Sarah Jane Harrison. “Subjective and Physiological Effects of Oromucosal Sprays Containing Cannabinoids (Nabiximols): Potentials and Limitations for Psychosis Research.” Current Pharmaceutical Design, vol. 18, no. 32, Dec. 2012, pp. 5008–5014., doi:10.2174/138161212802884708. Journal Impact Factor = 2.412, Times Cited = 6 (ResearchGate)
- Karschner, Erin L et al. “Plasma Cannabinoid Pharmacokinetics Following Controlled Oral Delta9-Tetrahydrocannabinol and Oromucosal Cannabis Extract Administration.” Clinical Chemistry, 57, no.1, 2011, pp. 66-75, doi:10.1373/clinchem.2010.152439. Journal Impact Factor = 8.008, Times Cited = 94 (ResearchGate)
- Zgair, Atheer et al. “Dietary Fats and Pharmaceutical Lipid Excipients Increase Systemic Exposure to Orally Administered Cannabis and Cannabis-based Medicines.” American Journal of Translational Research, 8, no. 8, 2016, pp. 3448-59. Journal Impact Factor = 2.829, Times Cited = 14 (ResearchGate)
- Birnbaum, Angela K., et al. “Food Effect on Pharmacokinetics of Cannabidiol Oral Capsules in Adult Patients with Refractory Epilepsy.” Epilepsia, vol. 60, no. 8, 2019, pp. 1586–1592, doi:10.1111/epi.16093. Journal Impact Factor = 5.562, Times Cited = 2 (ResearchGate)
- Ahn, Hyeji, and Ji-Ho Park. “Liposomal Delivery Systems for Intestinal Lymphatic Drug Transport.” Biomaterials Research, vol. 20, no. 1, 2016, doi:10.1186/s40824-016-0083-1. Journal Rank = 0.828, Times Cited = 12 (ResearchGate)
- Zgair, Atheer, et al. “Oral Administration of Cannabis with Lipids Leads to High Levels of Cannabinoids in the Intestinal Lymphatic System and Prominent Immunomodulation.” Scientific Reports, vol. 7, no. 1, June 2017, doi:10.1038/s41598-017-15026-z. Journal Impact Factor = 4.525, Times Cited = 10 (ResearchGate)
- Bruni, Natascia, et al. “Cannabinoid Delivery Systems for Pain and Inflammation Treatment.” Molecules, vol. 23, no. 10, 2018, p. 2478, doi:10.3390/molecules23102478. Journal Impact Factor = 3.060, Times Cited = 3 (ResearchGate)
Originally: The Reality of Oil Bioavailability, V1, V2, 2019
[Graphic 1: Public Domain]

















Recent Comments